AAMI’s New Cleaning Standards Reduce Endoscope Infection Risk
Josette Weinstein, Marketing Coordinator
In February and March of 2015, hospitals were hurrying to address a deadly antibiotic-resistant superbug that threatened North Carolina, Pittsburgh, Los Angeles, Chicago and Seattle. According to Charles Patrick David, MD, this superbug, Carbapenem-resistant Enterobacteriaceae (CRE), is a bacterial infection that has a prognosis of death in 40%-50% of infected patients. These infections were linked to a complex type of endoscope that was not properly decontaminated.
The culprit, in each case, was found to be a specialized endoscope called duodenoscopes. This particular endoscope is threaded down the throat to treat digestive disorders such as gallstones and cancers. Due to the duodenoscope’s complex structure, it often accumulates bacteria that are not always removed by conventional cleaning so infections are easily passed between patients. Regardless of these infection risks, endoscopes are important pieces of hospital equipment that often saves lives. It is critical for patient health that these scopes remain available due to the benefits often times outweighing the risks.
New Standards for Endoscope Reprocessing
Coming at a time when public concern about the cleanliness and decontamination of endoscopes and similar devices is at an all-time high, the Association for the Advancement of Medical Instrumentation (AAMI) has created a standard to the cleaning and reprocessing of endoscopes. This standard, titled: ANSI/AAMI ST91:2015, Comprehensive guide to flexible and semi-rigid endoscope processing in healthcare facilities, goes into extensive detail about the processes of cleaning, sterilizing, and disinfecting endoscopes. The standard also describes endoscope pre-cleaning, leak-testing, packaging and storage which were often left to each individual healthcare facility’s unique operating procedures.
Improve Patient Safety with Real-Time Locating Technology
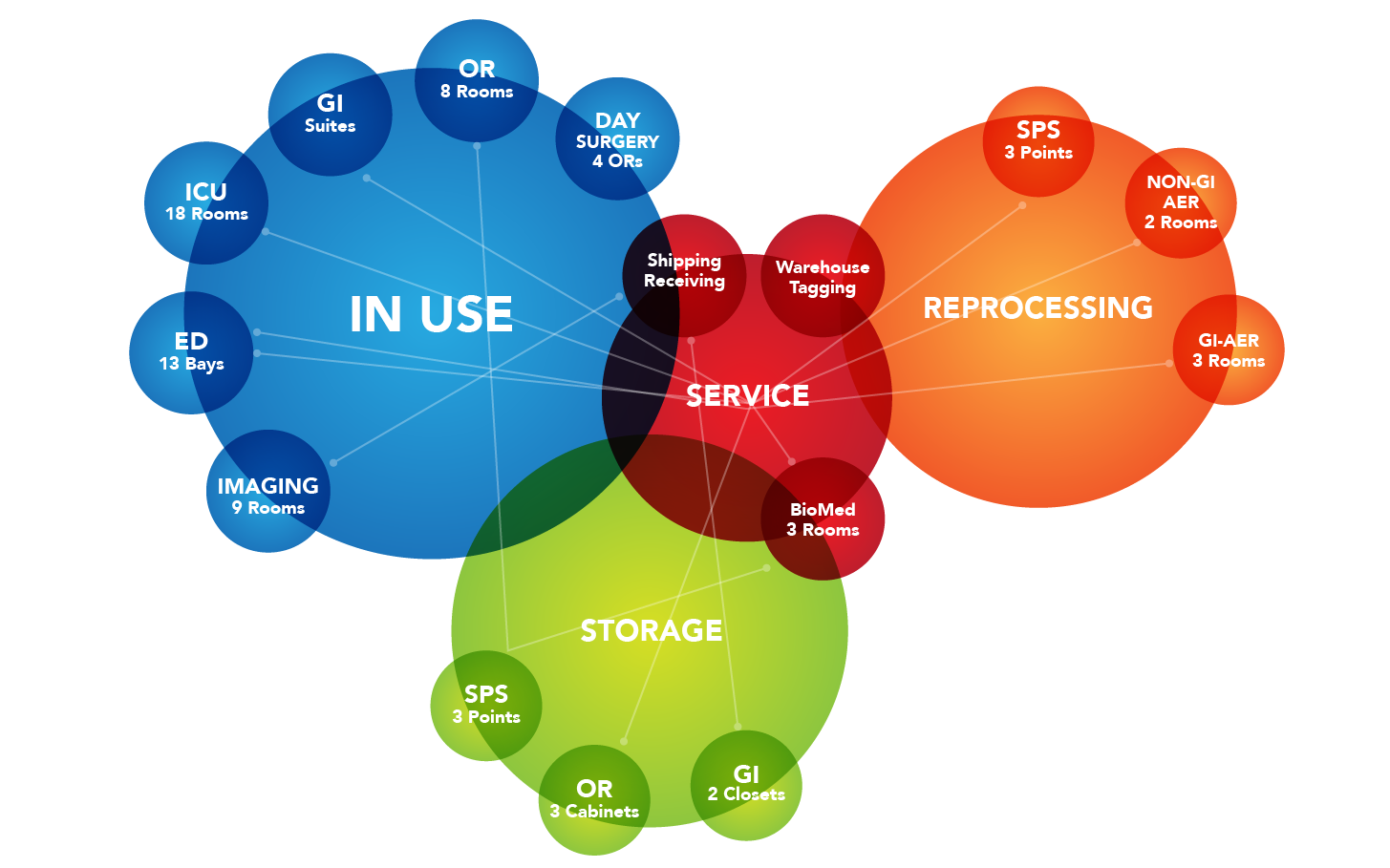
With the potential for wide-spread disease and the risk of infection with these devices, it’s crucial for healthcare facilities to have the ability to track the storage, usage and cleaning lifecycles of every endoscope. By using real-time locating technology, a healthcare facility is able to easily and safely monitor scopes through their usage, cleaning and storage lifecycles. Miniature, durable tags capable of withstanding High-Level Disinfection (HLD) can significantly improve patient safety by reducing infection risk.
One of the most important steps healthcare facilities must take is the reprocessing and storage of endoscopes when they aren’t being used. In order to avoid risk from organic matter or bioburden left on the endoscope, it’s important for these endoscopes to enter the first stage of reprocessing within a very short period. A Clinical-Grade Real-Time Location System (RTLS) would be able to track and create alerts through every stage of this cleaning process. Staff could know when endoscopes need to be cleaned and could immediately be notified of any possible infection risks.
To avoid unwanted bacteria growth and infection risk, all endoscopes need to be cleaned regularly. Clinical-Grade RTLS systems could enable facilities to know which scopes should be used first in order to maximize workflow and avoid unnecessary washing and disinfection that can be harmful to its structure.






